Ionic Magnetic Resonance Tailors Animal Cells/Tissues
health medicine and biotechnology
Ionic Magnetic Resonance Tailors Animal Cells/Tissues (MSC-TOPS-100)
Design and method allows culture regulation
Overview
Innovators at NASA Johnson Space Center have designed an apparatus and method that controls the growth and proliferation of 3D biological cells and mammalian tissue in the presence of a pulsating, alternating ionic magnetic resonance field (AIMR). The present technology applies a spectrum of electromagnetic fields to control the growth of all mammalian cells and tissues while simultaneously enabling cellular dedifferentiation and lifespan extension through the control of ionic transport and particular ion frequency resonances.
The innovation AIMR Multiple-Chambered, Apparatus for the Culture of Cells, Tissues and Organoid Bodies and Method of Use is at technology readiness level (TRL) 3 (which means analytical and experimental critical function and/or characteristic proof of concept has been derived) and the related issued patent is now available for your company to license.
The Technology
The apparatus comprises a randomized gravity vector multiphasic culture system with a self-feeding growth module, an optionally disposable nutrient module, and a removable AIMR chamber that delivers a pulsating multivariant field to the contents of the culture system. It produces overlapping or fluctuating alternating ionic magnetic resonance frequencies at one or more modal intervals ranging from about 7.8 Hz to about 59.9 Hz to the cell chamber. The apparatus may yield better regulation that can be manipulated to allow for increased rate of cell growth, faster differentiation, increased cell fidelity, and the induction or suppression of selective physiological genes involved in directing cellular differentiation and dedifferentiation.
The use of an AIMR field may provide a significant improvement over existing bioreactors, including pulsating electromagnetic field (PEMF) and time-variance electromagnetic field (TVEMF) cellular growth induced systems, in that AIMR incorporates the modulation of cellular transcription. The AIMR system utilizes pre-sterilized disposable modules and a removable alternating ionic magnetic resonance chamber, reducing the hazard for contamination, allowing scientists to implement physiological and homeostatic parameters similar to a naturally occurring physiological system.
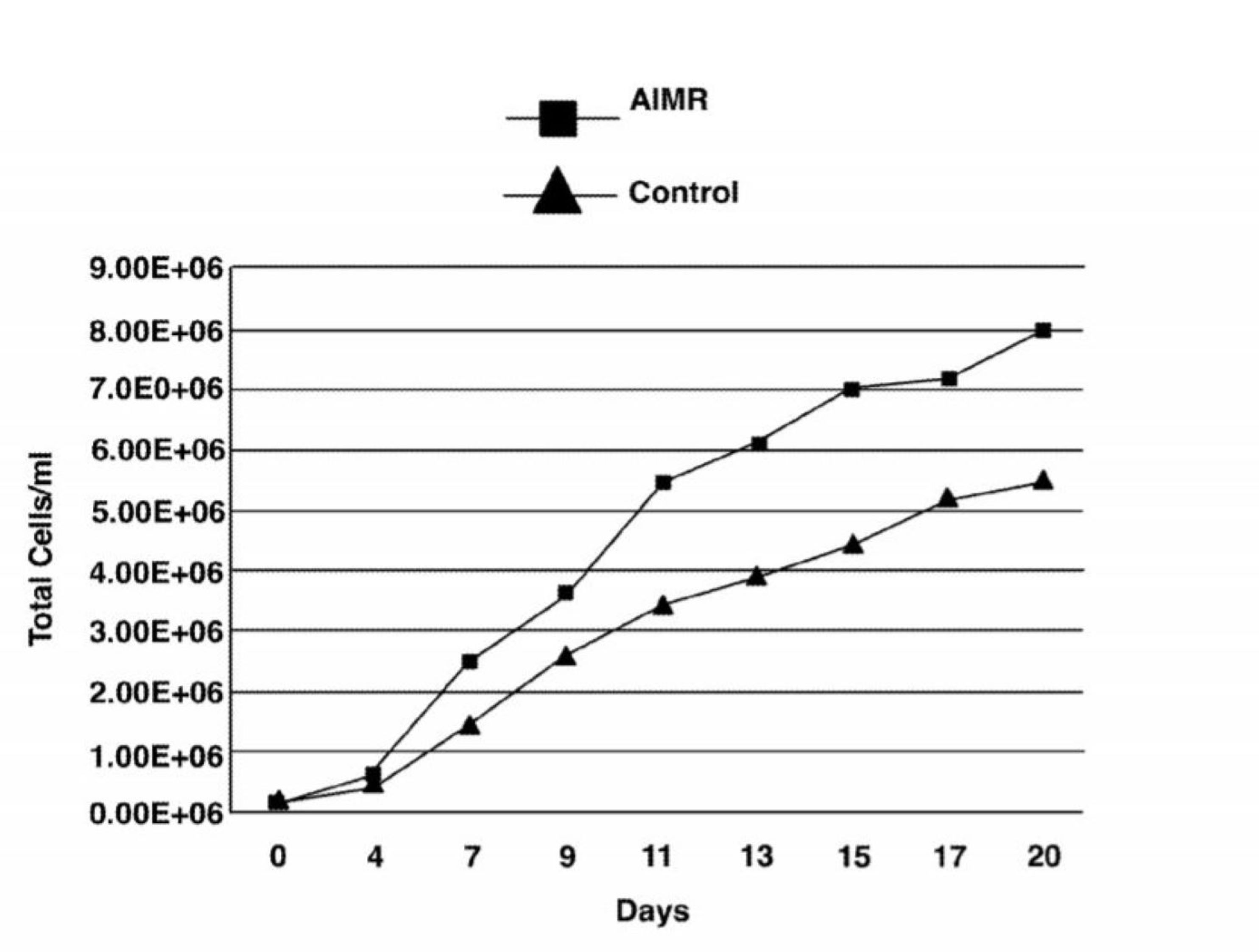
Benefits
- Can reduce reliance upon in vitro testing
- Enhanced control of cell differentiation/ dedifferentiation over prior art
- Pre-sterilized disposable device modules maintain homeostatic parameters
- Apparatus and method speeds optimized cell growth
Applications
- Medical: Vaccination studies for diseases; tissue replacement
- Cosmetics: Product development and testing
- Textiles: Testing physiological tolerance to substance
Similar Results

Micro-Organ Device Mimics Organ Structures for Lab Testing
The MOD platform technology represents a small, lightweight, and reproducible in vitro drug screening model that could inexpensively mimic different mammalian tissues for a multitude of applications. The technology is automated and imposes minimal demands for resources (power, analytes, and fluids). The MOD technology uses titanium isopropoxide to bond a microscale support to a substrate and uses biopatterning and 3D tissue bioprinting on a microfluidic microchip to eliminate variations in local seeding density while minimizing selection pressure. With the MOD, pharmaceutical companies can test more candidates and concentrate on those with more promise therefore, reducing R&D overall cost.
This innovation overcomes major disadvantages of conventional in vitro and in vivo experimentation for purposes of investigating effects of medicines, toxins, and possibly other foreign substances. For example, the MOD platform technology could host life-like miniature assemblies of human cells and the effects observed in tests performed could potentially be extrapolated more readily to humans than could effects observed in conventional in vivo cell cultures, making it possible to reduce or eliminate experimentation on animals.
The automated NASA developed technology with minimal footprint and power requirements, micro-volumes of fluids and waste, high throughput and parallel analyses on the same chip, could advance the research and development for new drugs and materials.

Noninvasive Therapy for Cartilage Regeneration
Research has shown that exposure of mammalian cartilage and bone tissue to tuned magnetic fields modifies genetic regulation at a cellular level. PEMF therapy relies on modulation and resonance of weak metals (ions) such as Ca2+, K+, Li+, and Mg2+ which can be made to move at the sub-cellular level when exposed to magnetic flux. This NASA technology is a device and method for modifying genetic regulation of cartilage and bone in response to PEMF therapy and may serve as the basis for development of novel therapies for cartilage diseases.
In initial studies, cultured human chondrocyte cells (HCH) from patients with early-stage osteoarthritis were exposed to PEMF stimulation using a variety of tuned electro-magnetic pulse characteristics such as flux magnitude, slew rates, rise and fall times, frequency, wavelength, and duty cycle. Waveforms used in testing were monophasic, bi-phasic, square, sinusoidal, and triangular in nature. Frequencies were generally low, ranging from 6-500 Hz, and the waveforms used high rising and falling slew rates on the order of Tesla/sec, promoting pulses or bursts.
Cellular catabolic and anabolic gene expression analyses comprised of fold-change (in expression) were accomplished by a survey of 47,000 human genes using an AFFYMETRIX Gene Array. Results show that variation of waveform used in PEMF therapies, independent of flux intensity, influences genetic regulation of HCH from patients with early-stage osteoarthritis.

Bio-Magnetic Device To Enhance Mammalian Tissue Repair
Most magnetic therapy research and resulting devices have centered around pulsed unidirectional bioelectric systems. The technology available here for licensing utilizes a square-wave time-varying electrical current, which generates an electromagnetic field, via a wound coil incorporated into a sleeve and encircles the affected appendage. An external and commercially available time-varying compact electrical generator connects to the wound coil within the sleeve and is powered by a 9-volt battery.
Prior industry attempts to use electromagnetic therapy on mammalian tissue have historically applied higher than necessary levels of electromagnetism, typically at 50 gauss or more. Researchers found that by inducing a Fourier-curve, time-varying electromagnetic wave at levels within 0.05 0.5 gauss for a pre-determined time-period, was optimum to achieve successful mammalian tissue regeneration.
It is theorized that magnetic fields can alter the flow of positively charged calcium ions that interact with the muscles around small blood vessels causing them to relax. This effect in turn, causes constricted blood vessels to dilate, and dilated blood vessels to constrict. Depending upon the type of injury, enhanced tissue repair may occur through the suppression of inflammation, or the increase in blood flow.

3D Mineralized Bone Constructs
One of the central objectives of this project was the development and characterization of a 3D mineralized tissue model system in which the effects of mechanical load (e.g., compression loading, tension, vibration, etc.) on the cellular responses of osteoblasts and osteoclasts could be investigated. After introducing mineralization agents to the culture, the constructs take on a bone-like appearance and have a more rigid structure suitable for being tested. Testing of the mineralized constructs confirmed the presence of calcium through a crystalline matrix histochemical stain. The central core is void of necrotic material, instead filled by a crystalline matrix with embedded nucleated cells. Remarkably, the nucleated cells do not express osteoblast markers, indicating differentiation to the in vivo cell type known as the osteocyte. In addition, as is characteristic to native periosteum, osteoclast precursor cells were imaged and proven to naturally arrange as an outer layer of the mineralized bone tissue construct. Development of this model will provide a unique venue for testing proposed countermeasures to space flight-induced bone loss. It will also allow a mechanistic approach in the modulation of cell signaling at the cellular level within the bone matrix.
The Development And Characterization Of A Three-Dimensional Tissue Culture Model Of Bone is a technology readiness level (TRL) 6 (system/subsystem prototype demonstrated in a relevant environment). The innovation is now available for your company to license. Please note that NASA does not manufacture products itself for commercial sale.

Electroactive Scaffold
Current scaffold designs and materials do not provide all of the appropriate cues necessary to mimic in-vivo conditions for tissue engineering and stem cell engineering applications. It has been hypothesized that many biomaterials, such as bone, muscle, brain and heart tissue exhibit piezoelectric and ferroelectric properties. Typical cell seeding environments incorporate biochemical cues and more recently mechanical stimuli, however, electrical cues have just recently been incorporated in standard in-vitro examinations. In order to develop their potential further, novel scaffolds are required to provide adequate cues in the in-vitro environment to direct stem cells to differentiate down controlled pathways or develop novel tissue constructs. This invention is for a scaffold that provides for such cues by mimicking the native biological environment, including biochemical, topographical, mechanical and electrical cues.



