Biomarker Sensor Arrays for Microfluidics Applications
sensors
Biomarker Sensor Arrays for Microfluidics Applications (NPO-TOPS-14)
For multi-color imaging and processing of single-molecule life signatures
Overview
NASA's Jet Propulsion Laboratory (JPL) offers a method to manufacture biomarker sensor arrays with nanoscale resolution and active regions on the order of 1 micron, by applying nanolithographic direct-write techniques to the fabrication of silane chemistry sensors on a transparent substrate. This novel technology enables extremely fine patterns of detectors suitable for multicolor imaging of single-molecule samples at resolutions far below the diffraction limit. The extremely small size of these sensors allows for rapid, highly specific screening for hundreds of functionalities within a single, small, integrated microfluidics chip.
The Technology
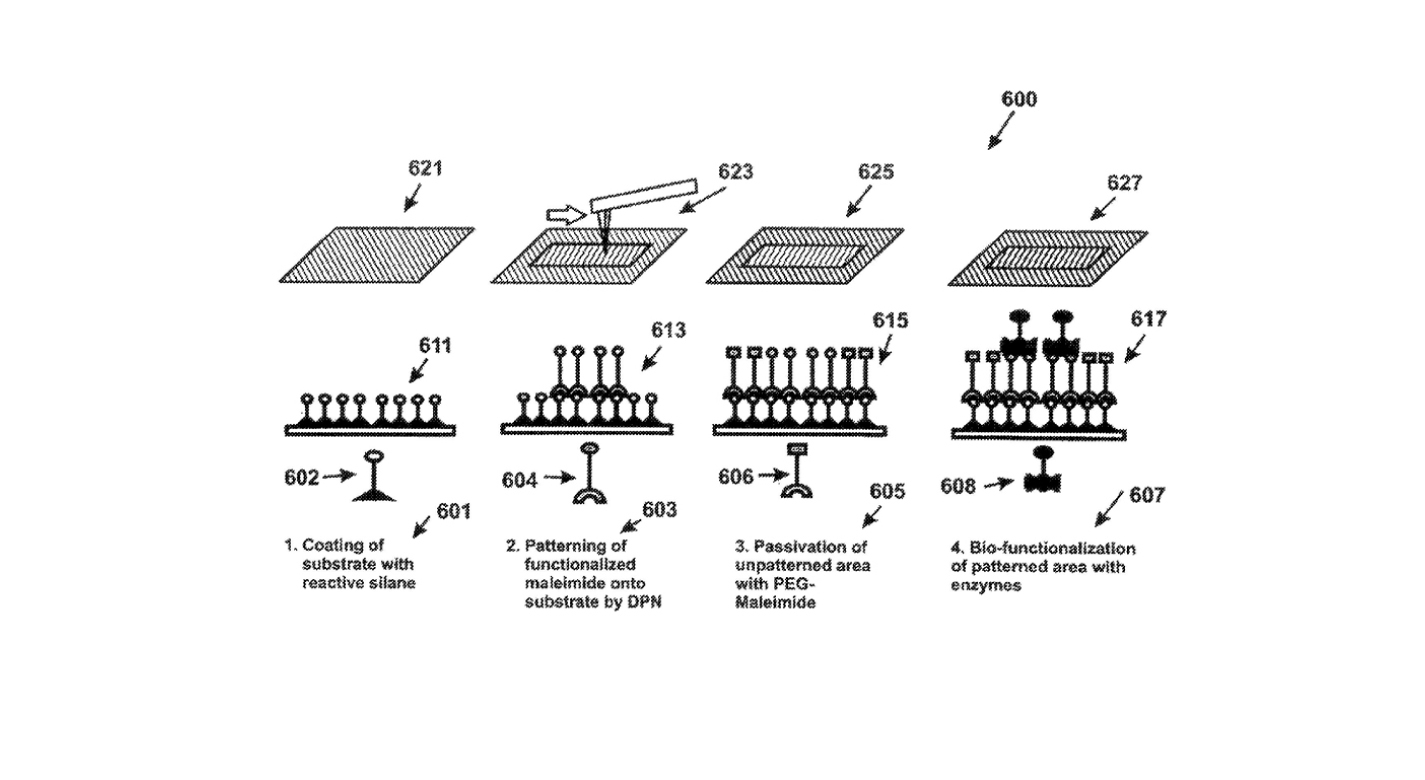
This invention provides a method and system for fabricating a biomarker sensor array by dispensing one or more entities using a precisely positioned, electrically biased nanoprobe immersed in a buffered fluid over a transparent substrate. Fine patterning of the substrate can be achieved by positioning and selectively biasing the probe in a particular region, changing the pH in a sharp, localized volume of fluid less than 100 nm in diameter, resulting in a selective processing of that region. One example of the implementation of this technique is related to Dip-Pen Nanolithography (DPN), where an Atomic Force Microscope probe can be used as a pen to write protein and DNA Aptamer inks on a transparent substrate functionalized with silane-based self-assembled monolayers. But it would be recognized that the invention has a much broader range of applicability. For example, the invention can be applied to formation of patterns using biological materials, chemical materials, metals, polymers, semiconductors, small molecules, organic and inorganic thins films, or any combination of these.
Benefits
- Fast: Rapidly interrogates, with high selectivity, very sparse samples within microfluidic systems (e.g., less than 10 microseconds)
- Precise: Offers better resolution at smaller sizes (~1 micron or less)
- Versatile: Can apply patterns using a wide range of previously incompatible materials (e.g., biologicals, chemicals, metals, polymers, small molecules dendrimers, proteins, semiconductors, insulators, thin films, etc.) and in various pH environments
- Efficient: Carries out sequential reactions with improved throughput and greater yield
Applications
- Life sciences - medical diagnostic systems, pharmaceutical research, biotechnology
- Security - detection of toxins and bio-weapons
- Agriculture - processing and analysis of soil samples
- Chemistry - organic and inorganic, molecular interactions
- Electronics and semiconductors
- Petroleum
Similar Results

Microfluidics Pressure-Based Switching and Valving Array
The innovative technology from Ames acts as a microfluidics switch array, using combinations of pressure and flow conditions to achieve specific logic states that determine the sequences of sample movement between microfluidic wells. This advancement will enable automation of complex laboratory techniques not possible with earlier microfluidics technologies that are designed to follow predetermined flow paths and well targets. This novel method also enables autonomous operations including changing the flow paths and targeting well configurations in situ based on measured data decision parameters. This microfluidic system can be reconfigured for use in various experimental applications, requiring only an adjustment of the programmed pressure sequencing, reducing the need for custom design and development for each new application. For example, this technology could provide the ability to selectively constrain or move biological specimens in the experimental wells, allowing evolutionary studies of model organisms in response to various stressors, evaluation of different growth conditions on biological production of antibodies or other small molecule therapeutics, among other potential applications. Likewise, this platform can be used to foster time-dependent, step-wise, chemical reactions, which could be used for novel chemical processes or in situ resource utilization.

Electrochemical Sensors Based on Enzyme-Linked Immunosorbent Assay
NASA’s electrochemical Enzyme-Linked Immunosorbent Assay (ELISA) microelectrode array biosensor advantageously incorporates a microbead detection construct, coupled with a magnetic immobilization construct, which substantially increases the signal sensitivity of a sensor. The magnetic immobilization construct draws the microbead detection construct to an electrode detection surface, enhancing signal sensitivity. By concentrating the signaling molecules close to the electrode detection surface, electrochemical redox cycling is achieved by reducing the distance between the two, allowing for regeneration of reporter molecules.
Whereas a traditional ELISA testing exhibits five to ten signaling molecules per probe molecule binding event, the present electrochemical ELISA-based biosensor testing exhibits up to 4,857 signaling molecules per probe molecule binding event. The model bead construct exhibits a more than 6.75-fold in increased measured signal, and more than 35.7-fold improvement in signal sensitivity. When compared to traditional optical ELISA, the present invention improves the limit of detection by up to a factor of 60.5.
NASA’s electromagnetic ELISA-based biosensor can be used for the detection of SARS-CoV-2 virus to enhance Covid-19 testing during the early phases of infection. The technology may also be modified to detect other biomarkers.

Nanosensor Array for Medical Diagnoses
Many diseases are accompanied by characteristic odors. Their recognition can provide diagnostic clues, guide the laboratory evaluation, and affect the choice of immediate therapy. The study of the chemical composition of human breath using gas chromatography mass spectrometry (GC/MS) has shown a correlation between the volatile compounds and the occurrence of certain illnesses. The presence of those specific compounds can provide an indication of physiological malfunction and support the diagnosis of diseases. This condition requires an analytical tool with very high sensitivity for its measurement. A number of volatile compounds, so called biomarkers, are found in breath samples, normally at low parts per billion (ppb) levels. For example, the acetone in the exhaled breath from human with other biomarkers can indicate Type I diabetes. Usually, the concentration of the volatile compounds in human breath is very low and the background relative humidity is high, almost 100%. NASAs invention utilizes an array of chemical sensors combined with humidity, temperature, and pressure for real-time breath measurement to correlate the chemical information in the breath with the state and functioning of different human organs. This tool provides a non-invasive method for fast and accurate diagnosis at the medical point of care or at home. The sensor chip includes multisensors for a comprehensive measurement of chemical composition, temperature, humidity, and pressure/flow rate. The sensor data collected from this chip can be wired or wirelessly transmitted to a computer terminal at the doctors desk or hospital monitoring center. The sensor chip can be connected directly or via Universal serial bus (USB) to a cell phone for data transmission over a long distance and receive an instruction from a doctors office for an immediate therapy.

Hybrid carbon nanotube-gold nanoparticle composite for Nitric Oxide (NO) detection
A hybrid thin film is fabricated by a simple drop-casting method. Functionalized single-walled carbon nanotubes (SWCNTs) and gold nanoparticles (AuNPs) with a diameter of ≈15 nm are drop-casted onto a printed circuit board (PCB) substrate equipped with interdigitated electrodes. The addition of AuNPs to the carbon nanotube networked films enhance sensitivity and lower the detection limit to low parts-per-billion (ppb) concentrations. The gold particle to carbon nanotube ratio is optimized to find the optimum gold nanoparticle loading.
The composite films were tested in both air and nitrogen environments across a wide relative humidity range (0-97%), which is suitable for dissolved Nitric Oxide (NO) detection in sea water for oceanographic study and for human breath analysis in medical diagnosis. The sensors exhibited high selectivity, particularly to NO, outperforming other tested gases. Notably, the sensor reliably detected NO at 10 ppb levels with response times within 10 seconds and recovery time around 1 minute, showcasing excellent reproducibility across sensors and operational efficiency within diverse humidity conditions.

Micro-Organ Device Mimics Organ Structures for Lab Testing
The MOD platform technology represents a small, lightweight, and reproducible in vitro drug screening model that could inexpensively mimic different mammalian tissues for a multitude of applications. The technology is automated and imposes minimal demands for resources (power, analytes, and fluids). The MOD technology uses titanium isopropoxide to bond a microscale support to a substrate and uses biopatterning and 3D tissue bioprinting on a microfluidic microchip to eliminate variations in local seeding density while minimizing selection pressure. With the MOD, pharmaceutical companies can test more candidates and concentrate on those with more promise therefore, reducing R&D overall cost.
This innovation overcomes major disadvantages of conventional in vitro and in vivo experimentation for purposes of investigating effects of medicines, toxins, and possibly other foreign substances. For example, the MOD platform technology could host life-like miniature assemblies of human cells and the effects observed in tests performed could potentially be extrapolated more readily to humans than could effects observed in conventional in vivo cell cultures, making it possible to reduce or eliminate experimentation on animals.
The automated NASA developed technology with minimal footprint and power requirements, micro-volumes of fluids and waste, high throughput and parallel analyses on the same chip, could advance the research and development for new drugs and materials.



